FAQs | Everything You Need to Know About Formic Acid
Formic acid, also known as methanoic acid, is a simple yet crucial chemical in various industries. This article addresses frequently asked questions about formic acid, shedding light on its properties, uses, hazards, and handling precautions.
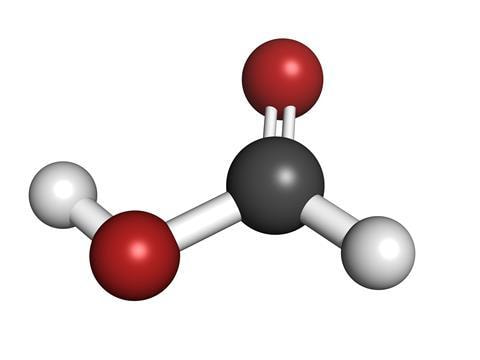
What is Formic Acid?
Formic acid is the simplest carboxylic acid, known chemically as HCOOH or CH₂O₂. Naturally occurring in ants, its name derives from "formica," the Latin word for ant. It can also be synthesized industrially from methanol.
What is Formic Acid Used For?
Formic acid has a wide range of applications across different industries, including:
Agriculture: Used in the production of pesticides.
Leather Industry: Essential for leather tanning.
Textile Industry: Utilized in dye-making, fabric processing, textile printing, and dyeing.
Industrial Applications: Acts as a solvent, surface treatment agent, and rubber auxiliary.
Animal Feed: Used as an antibacterial agent in livestock feed.
Preservation: Employed in various preservation processes.
Soldering: Enhances solder wettability during the soldering process.
What is the Chemical Formula of Formic Acid?
The chemical formula of formic acid is CH₂O₂.
Is Formic Acid Harmful to Life?
Formic acid poses significant health risks. It is acutely toxic to humans, causing severe irritation to the skin, eyes, and respiratory system. Prolonged exposure can lead to serious health issues.
Where is Formic Acid Found, and What Does It Look Like?
Formic acid is naturally present in ants, stingless bees, and puss moth caterpillars. Industrially, it is produced by reacting methanol with carbon monoxide in the presence of sodium methoxide. It is a colorless liquid with a pungent odor and a fuming appearance. It is soluble in water, ether, acetone, and methanol.
What Are the Hazardous Effects of Formic Acid?
Formic acid is hazardous and has the following effects:
Additional reading:Can Silica Fume Enhance the Magic of Ceramics?
Skin Corrosion: Can cause irreversible damage and severe burns.
Eye Irritation: May lead to serious eye damage if contact occurs.
Ingestion: Harmful if swallowed.
Combustibility: Formic acid is a flammable substance.
What Precautionary Measures Are Necessary to Handle Formic Acid?
Proper precautions are essential when handling formic acid. Follow these safety measures:
Protective Gear: Wear gloves, an apron, and protective footwear.
Eye Protection: Use safety glasses or eye protection.
Respiratory Protection: Utilize respiratory masks to avoid inhaling fumes.
Avoid Consumption: Do not eat, drink, or smoke while handling formic acid.
Hygiene: Wash hands and body thoroughly after handling.
Fire Safety: Keep formic acid away from heat, sparks, hot surfaces, and flames.
What Will Happen If Formic Acid Is Heated?
When heated, formic acid can catch fire as it is combustible. It should be stored and handled away from heat sources to prevent accidents.
Is Formic Acid a Soluble Compound?
Yes, formic acid is soluble in water, ether, acetone, and methanol.
Where Can I Buy Formic Acid?
Formic acid is available for purchase from various suppliers, including TJCY. They offer different packaging options, such as 10 metric tons and 20 metric tons, and ship chemicals worldwide.
Conclusion
Formic acid is a versatile chemical with numerous industrial applications, from agriculture to textiles and animal feed. Despite its usefulness, it is essential to handle it with care due to its hazardous nature. By understanding its properties, uses, and safety measures, you can safely and effectively utilize formic acid in various applications. For reliable supply and quality, consider purchasing from reputable suppliers like TJCY.